Recently, the rapid development of electronic technology towards high frequency raises a great demand of various inductive devices for very high frequency (VHF). As a result, corresponding soft magnetic materials becomes more and more attractive in both academic and industrial areas.
Among many ferrites, M-type barium (BaM) hexaferrite (BaFe12O19) is a well known hard magnetic material with strong magnetocrystalline anisotropy along c-axis. However, many reports revealed that proper substitution on Fe3+ can change the anisotropy from uniaxial to planar, and endow it excellent soft magnetic properties, such as higher saturation magnetization than Y-type (one kind of hexagonal ferrite). Hence, Co–Ti substituted M-type hexaferrite is a promising soft magnetic material in VHF.
In addition, low-firing is another important performance of soft magnetic ferrites for the applications in multilayer chip devices and components, because ferrite should be cofired with metal inner electrode. To lower the sintering temperature of ferrite, Bi2O3 was often used as a sintering additive. Besides, some researchers also used Bi3+ to substitute Fe3+ in BaM hexaferrite. In this research, Bi in Co–Ti substituted M-type hexaferrite is introduced and the effect of Bi–Co–Ti substitution on the phase formation, sintering behavior, and magnetic properties of BaM hexaferrite is investigated.
For the experimental procedure, Bi–Co–Ti substituted BaM ferrite of Ba1 − xBix(Co1.1 + xTi1.1)Fe9.8 − xO19 (x = 0.05–0.3) was prepared by solid-state reaction method. The starting materials, reagent grade Fe2O3, BaCO3, Bi2O3, Co3O4 and TiO2, were mixed for 4 h by a planetary ball-mill. The mixture was calcined at 900 °C for 4 h, and then ground again for 4 h. The resultant powders were dry-pressed in stainless steel die. The pressed pellet and toroidal samples were sintered in the temperature range from 900 °C to 1100 °C for 6 h in air.
And then, the phase composition was characterized by X-ray diffraction (XRD). The density of the sintered samples was determined by the Archimedes’ method. The microstructure of the fracture surface of the sintered samples was observed by scanning electron microscope (SEM). The complex permeability was measured using an Agilent E4991A RF impedance/materials analyzer from 1 MHz to 1 GHz with the 16454A test fixture.
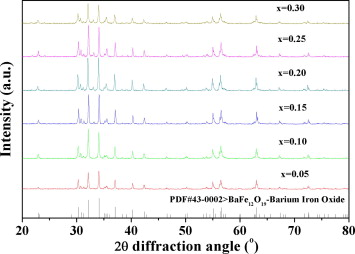
Fig. 1. XRD patterns of Ba1 − xBix(Co1.1 + xTi1.1)Fe9.8 − xO19 calcined at 900 °C for 4 h (Image by the University of Science and Technology Beijing).
Fig. 1 shows the XRD spectra of the samples calcined at 900 °C. It shows that the characteristic diffraction peaks in each spectrum correspond to the M-type hexaferrite structure with P63/mmc.
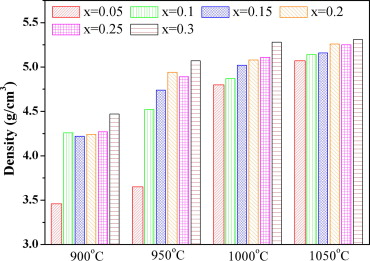
Fig. 2. Density of Ba1 − xBix(Co1.1 + xTi1.1)Fe9.8 − xO19 sintered at different temperatures (Image by the University of Science and Technology Beijing).
Fig. 2 shows the density of Ba1 − xBix(Co1.1 + xTi1.1)Fe9.8 − xO19 (x = 0.05–0.30) sintered at different temperatures. In general, the density increases with the rise of Bi amount or sintering temperature.
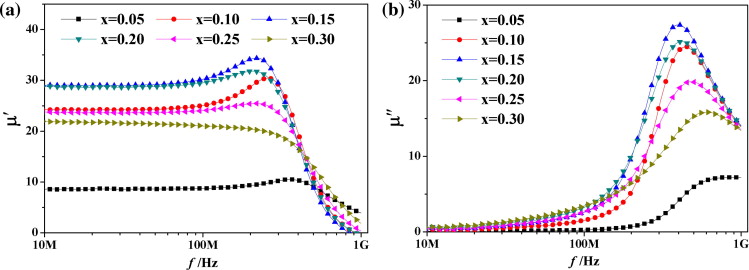
Fig. 3. Complex permeability of Ba1 − xBix(Co1.1 + xTi1.1)Fe9.8 − xO19 sintered at 950 °C (Image by the University of Science and Technology Beijing).
Fig. 3. shows the frequency dependence of the complex permeability of the samples sintered at 950 °C.
Shown in the experiment, Bi–Co–Ti substituted M-type hexaferrite of Ba1 − xBix(Co1.1 + xTi1.1)Fe9.8 − xO19 (x = 0.05–0.3) is successfully prepared by solid-state reaction method. The single-phased M-type hexaferrite is obtained at 900 °C. Bi substitution effectively promotes the sintering densification of M-type hexaferrite by liquid phase, so the samples with x > 0.1 can be sintered well at 950 °C. Moreover, Bi substitution remarkably improves the soft magnetic properties in very high frequency (VHF). In addition, the low sintering temperature facilitates its application in multilayer chip devices.
This research result was published online: http://www.sciencedirect.com/science/article/pii/S0925838812023560 and was published on the currently issued Journal of Alloys and Compounds (Vol. 556, Pages 20–25, 15 April, 2013).


